Cystic Fibrosis Community Care is calling on our community to join us in demanding #TrikaftaNOW
UPDATE: APRIL 2023
The Minister for Health, the Honorary Mark Butler, announced that Trikafta will be subsidised on the PBS from 1st May 2023, for 6-11-year-olds with at least one F508del mutation.
There are more than 500 Australian children, including approximately 260 in New South Wales and Victoria that will benefit from this monumental announcement.
We know it has been an emotional roller-coaster with the unexpected referral back to the PBAC in the March meeting regarding the compliance and adherence metrics. Today we can be assured that soon more people will be eligible for Trikafta.
"We are delighted with the news that Trikafta will be available on the PBS for eligible Australian children aged 6-11 from May 1, 2023. This has been a community effort and we thank everyone from our community who shared their stories and supported the advocacy efforts." - Andre Carvalho, CEO Cystic Fibrosis Community Care.
Your voice has been heard.
Thank you as well to the Health Minister, the Department of Health, and Vertex Pharmaceuticals for accelerating these discussions.
May 1st cannot come soon enough!
"Whilst this is a very positive step forward, there is still a long way to go to ensure everyone can have access to effective therapies and this includes people with rare mutations, people who are not eligible for current therapies, or who do not respond to modulators. This work continues with urgency." says Andre.
We will continue to keep you updated on our advocacy efforts.
Survival rates and quality of life for people with Cystic Fibrosis is improving dramatically with the emergence of new, lifechanging medications.
One of these therapies is Trikafta, which last April was approved in Australia for those aged 12 years and older who have the most common CF gene mutation. Now, we are trying to have Trikafta approved for those aged 6-11 years old as well.
There have been several delays in this process and children living with CF cannot wait any longer.
The Power of Community
This is where the power of community makes a difference. We encourage everyone to take action, whether you are living with Cystic Fibrosis or simply want to use your voice to support the community.
Every action helps.
In 3 simple steps you can help us to take action.
1. Download #TrikaftaNOW tiles to share through social media
Simply RIGHT click and save any of the below images to share.




Below is a suggested post you can write if you're not sure what to say. However your story will always be more powerful, so share why you care.
Trikafta is needed NOW for children aged 6-11.
500 Australian children cannot wait any longer.
#TrikaftaNOW
Make sure to tag:
@cfcommunitycare on Instagram
@CFCCAUST on Facebook
and @markbutlerMP
2. Download this draft template letter, complete with your personal story and email to Mark Butler MP
DOWNLOAD TEMPLATE
3. Share your story
It's powerful to share stories, and important for people to see the people who are impacted.
Make sure to tag:
#TrikaftaNOW
@cfcommunitycare on Instagram
@CFCCAUST on Facebook
and @markbutlerMP
Campaign information and updates on progress will be posted on this webpage and social media.
Facebook - Instagram - LinkedIn - Twitter
TRIKAFTA. What has happened so far?
Last December, the PBAC recommended Trikafta for ages 6-11 years to be listed on the PBS.
The final decision to list on the PBS then sat with the Federal Government; however, discussions between Vertex and the Health Department came to a standstill, and the matter was referred back to the PBAC for consideration at their March meeting.
The crux of the dispute appears to be regarding metrics about uptake and compliance rates, which is the number of children who are likely to start treatment and those who will continue to use it. This is surprising given that there is significant data on this.
Earlier this month, the PBAC met as planned and we, the general public, won’t be notified of the final decision until the 21st April, when the outcome of the meeting will be made public.
We hope that the PBAC provides a positive outcome, which then allows the Health Department to resume its work to list on the PBS. This process must not be delayed any further, and we call Mark Butler MP, Minister of Health to fast track the listing of Trikafta as soon as possible.
This news has come at a time when our neighbours in New Zealand are celebrating that Trikafta will be available for those eligible with CF aged 6 and older from April 1st, 2023. This is great news for New Zealand. However, it is disappointing to think that two countries so close have such significant inequity of access to this critical medication.
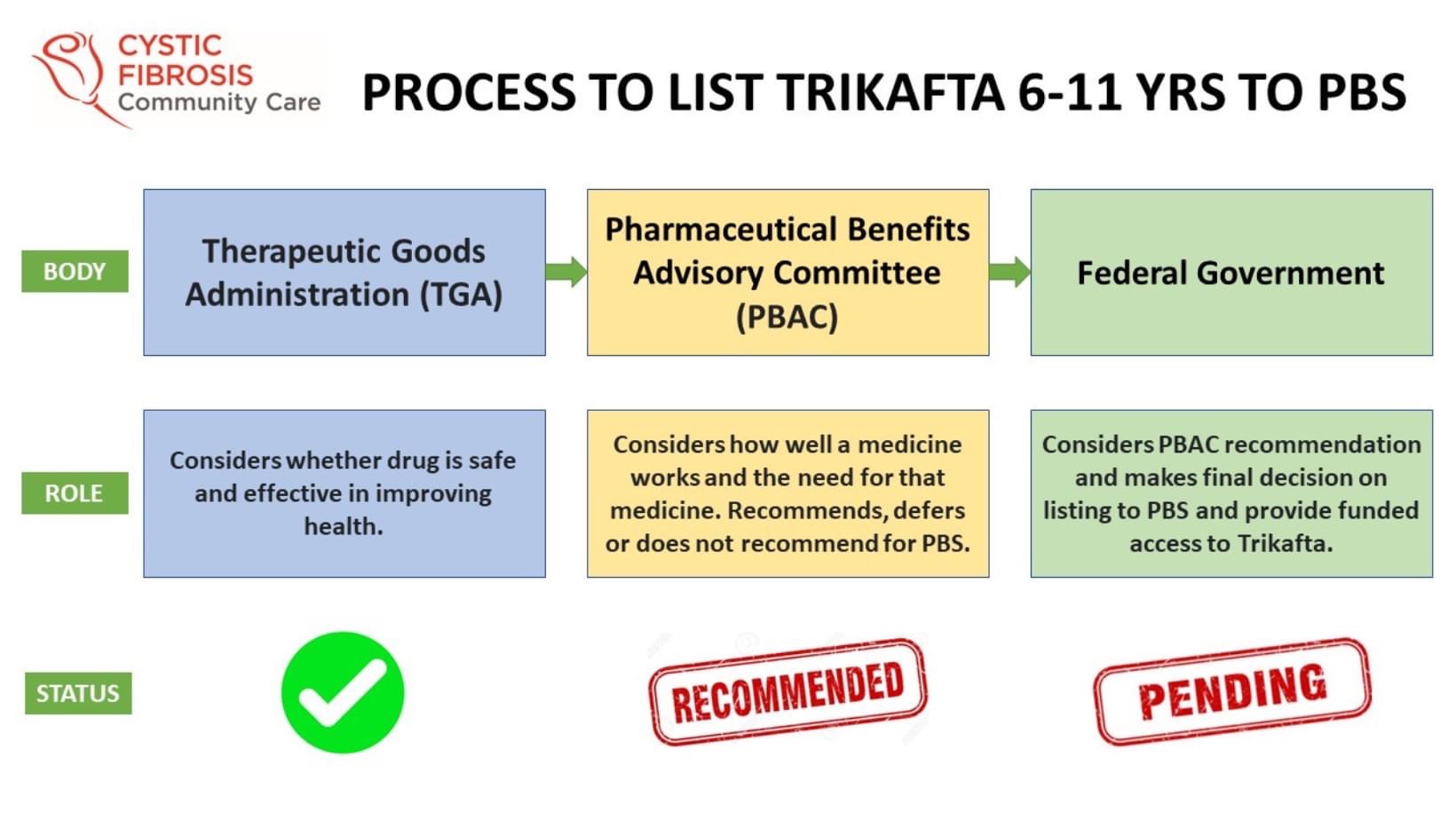
So what is the process to get new drugs, like Trikafta, available to patients?
Once new drugs have proven to be safe and successful in clinical trials, the pharmaceutical companies must apply to the Therapeutic Goods Administration, for approval to market the new treatment in Australia.
It is then evaluated by the Pharmaceutical Benefits Advisory Committee, or the PBAC, who take into consideration the clinical benefit and cost-effectiveness of the treatment.
The PBAC then decides to make a recommendation to the Australian Government for listing on the Pharmaceutical Benefits Scheme, or the PBS, so that it becomes available to Australians at an affordable price.
The final decision lies with the Federal Government (Health Minister) to consider the recommendation from PBAC and list on the PBS.